Enantioselective rearrangement of indolyl carbonates catalyzed by chiral DMAP-N-oxides†
Abstract
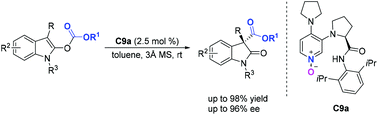
Bifunctional chiral DMAP-N-oxides have been reported for the highly enantioselective rearrangement of indolyl carbonates. Using 2.5 mol% of chiral DMAP-N-oxide, diverse oxindoles containing an all-carbon quaternary stereocenter were obtained in excellent yields (up to 98% yield) with high enantioselectivities (up to 96% ee). Compared to the widespread use of chiral DMAP and isothiourea catalysts, in which the nitrogen atom serves as a nucleophilic site, we found that chiral DMAP-N-oxides, which utilize the oxygen atom as a nucleophilic site, were also efficient organocatalysts in this rearrangement.


 Please wait while we load your content...
Please wait while we load your content...