Asymmetric organocatalyzed reaction sequence of 2-hydroxy cinnamaldehydes and acyclic N-sulfonyl ketimines to construct diverse chiral bridged polycyclic aminals†
Abstract
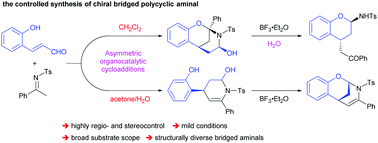
Asymmetric organocatalytic annulation reactions have been developed, where both 2-hydroxy cinnamaldehydes and acyclic N-sulfonyl ketimines were used as multisite substrates (more than two reactive sites) in an iminium catalysis triggered sequential process. Notably, H2O is crucial for reactivity and selectivity, since different isolable hemiaminal intermediates were obtained in aqueous medium, thus leading to diverse chiral bridged polycyclic aminals in a highly regio- and stereoselective manner. Several other interesting transformations with the obtained aminals were also realized.
- This article is part of the themed collection: 2019 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...