Access to polyfunctionalized carbazoles through π-extension of 2-methyl-3-oxoacetate indoles†
Abstract
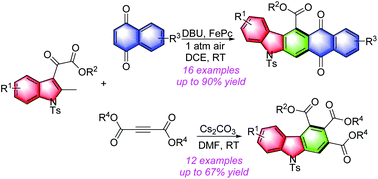
Formal [4 + 2] annulation of 2-methyl-3-oxoacetate indoles with naphthalene-1,4-dione and dialkyl acetylene dicarboxylates has been successfully developed. A structurally diverse set of polyfunctionalized carbazoles was efficiently synthesized in acceptable to excellent yields. This reaction features H2O as the only by-product, has a broad substrate scope and proceeds under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...