Expanstines A–D: four unusual isoprenoid epoxycyclohexenones generated by Penicillium expansum YJ-15 fermentation and photopromotion†
Abstract
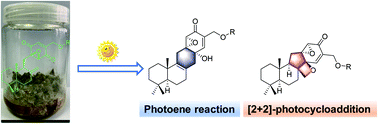
Two new isoprenoid epoxycyclohexenones, expanstines A–B (1–2), and two novel isoprenoid epoxycyclohexenones, expanstines C–D (3–4), featuring an unusual oxetane ring were isolated from Penicillium expansum YJ-15. Their structures were initially investigated in detail by NMR spectroscopy, HRESIMS, XRD and ECD experiments. Expanstines A–D were derived under UV and visible light from 5 and 6, which plausibly underwent a rare intramolecular photoene reaction and a [2 + 2] Paternò–Büchi photoaddition respectively. Both 3 and 4 exhibited potent antibacterial activities against B. subtilis, with an MIC of 0.25 μg mL−1.


 Please wait while we load your content...
Please wait while we load your content...