A highly efficient Hg(OTf)2-mediated Sakurai–Hosomi allylation of N-tert-butyloxycarbonylamino sulfones, aldehydes, fluoroalkyl ketones and α,β-unsaturated enones using allyltrimethylsilane†
Abstract
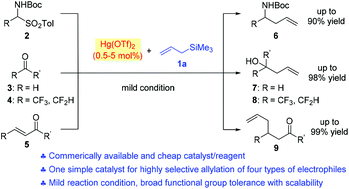
It is reported that cheap and easily available Hg(OTf)2 can efficiently mediate the Sakurai–Hosomi reaction of N-tert-butyloxycarbonyl (Boc) amino sulfones, aldehydes, and α-fluoroalkyl ketones using allyltrimethylsilane, with the catalyst loading down to 0.5–5.0 mol%, enabling the facile access to synthetically valuable homoallylic alcohols or amines, respectively. A chemoselective 1,4-allylation of α,β-unsaturated enones is also achieved under the catalysis of 5.0 mol% Hg(OTf)2. In most cases, Hg(OTf)2 exhibited intriguing properties superior to those of commonly used metal Lewis acids for such reactions, suggesting that mercury catalysis is worthwhile to explore.


 Please wait while we load your content...
Please wait while we load your content...