Transition metal-free aerobic oxidative cleavage of the C–N bonds of α-amino esters†
Abstract
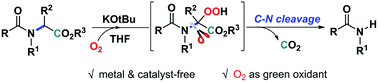
An efficient transition metal-free cleavage of the C–N bonds of α-amino esters under mild conditions has been developed. The reaction was performed in the presence of KOtBu and molecular oxygen. Mechanistic studies reveal that the reaction proceeds through a base-mediated aerobic oxidative cleavage pathway involving the formation of a hydroperoxide intermediate.


 Please wait while we load your content...
Please wait while we load your content...