Development of a new bicyclic imidazole nucleophilic organocatalyst for direct enantioselective C-acylation†
Abstract
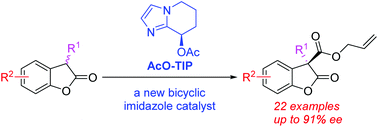
A novel chiral nucleophilic organocatalyst easily synthesized from simple starting materials bearing a 5,6,7,8-tetrahydroimidazo[1,2-a]pyridine skeleton has been developed and successfully applied in the direct enantioselective C-acylation of 3-substituted benzofuranones. Its catalytic efficiency was shown to be comparable to that of the previously reported chiral 6,7-dihydro-5H-pyrrolo[1,2-a]imidazole catalyst. A wide range of 3,3-disubstituted benzofuranones, possessing a quaternary stereocenter, were synthesized with high yields and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...