Mechanistic insights for the transprotection of tertiary amines with Boc2O via charged carbamates: access to both enantiomers of 2-azanorbornane-3-exo-carboxylic acids†
Abstract
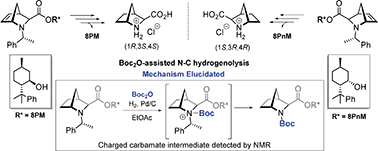
Herein we present a new synthesis methodology for the transprotection of the 1-phenylethyl protected tertiary amines to tert-butyloxycarbonyl (Boc) derivatives under exocyclic N–C hydrogenolysis catalyzed by Pd/C. We provide mechanistic insights into the N-dealkylation of hindered tertiary amines under the unrecognized role of tert-butyloxycarbonyl anhydride (Boc2O) as an additive to effectively promote the exocyclic N–C hydrogenolysis of tertiary amines by disclosing the role of Boc2O, which was not fully understood and studied until now. NMR and in silico experiments suggest the formation of a transient charged carbamate as the plausible intermediate. This selective transprotection enabled the development of a robust stereoselective methodology for the preparation of both enantiomers of 2-azanorbornane-3-exo-carboxylates by highly asymmetric aza-Diels–Alder reactions using (−)-8-phenylmenthol (8PM) and (+)-8-phenylneomenthol (8PnM) as chiral auxiliaries.


 Please wait while we load your content...
Please wait while we load your content...