Access to 2-substituted 1-pyridin-3-yl-β-carboline derivatives by intramolecular radical cyclization-ring opening-SNAr substitution†
Abstract
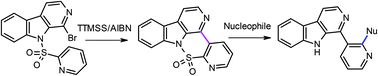
A new method for the synthesis of 1-pyridin-3-yl-β-carboline derivatives involving an intramolecular radical reaction in the presence of tris(trimethylsilyl)silane and azobisisobutyronitrile (TTMSS/AIBN), via a sultam intermediate, which proceeds as a one-pot process by ring opening and SNAr substitution, is described.


 Please wait while we load your content...
Please wait while we load your content...