A rhodium-catalyzed three-component reaction of arylisocyanides, trifluorodiazoethane, and activated methylene isocyanides or azomethine ylides: an efficient synthesis of trifluoroethyl-substituted imidazoles†
Abstract
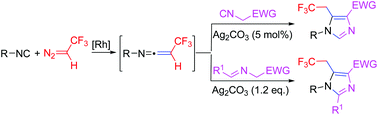
A rhodium-catalyzed three-component reaction of isocyanides, 2,2,2-trifluorodiazoethane and activated methylene isocyanides or azomethine ylides has been developed. In this transformation, the ketenimine intermediates generated by the coupling reaction of arylisocyanides with 2,2,2-trifluorodiazoethane undergo intermolecular [3 + 2] cyclization with activated methylene isocyanides or azomethine ylides and provide a new and highly efficient method for the construction of trifluoroethyl-substituted imidazoles in a single step.


 Please wait while we load your content...
Please wait while we load your content...