TfOH-promoted transformation of TMS-ethers of diarylsubstituted CF3-allyl alcohols with arenes into CF3-indanes†
Abstract
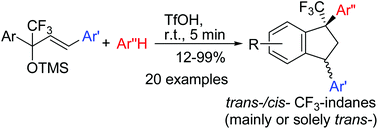
The reaction of TMS-ethers of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-ols with arenes in TfOH at room temperature for 5 min results in the formation of trans-/cis-1,3-diaryl-1-trifluoromethyl indanes in good yields (up to 99%). The predominant or exclusive formation of indanes with a trans-configuration of 1,3-diaryl groups has been observed. The reaction proceeds through an intermediate formation of the corresponding mesomeric CF3-allyl cations. A plausible reaction mechanism is discussed.


 Please wait while we load your content...
Please wait while we load your content...