A simple approach to indeno-coumarins via visible-light-induced cyclization of aryl alkynoates with diethyl bromomalonate†
Abstract
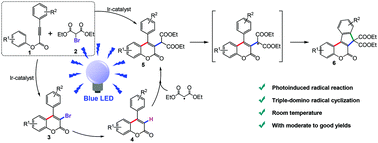
Visible-light-induced triple-domino cyclization between aryl alkynoates and diethyl bromomalonate was developed for the synthesis of indeno-coumarins. This one-pot method substantially simplified the production process for indeno-coumarins and a series of indeno-coumarins could be isolated in moderate to high yields. It was found that the obtained indeno-coumarins could be used as additional photosensitizers to initiate this transformation. A possible radical mechanism accompanying the processes of electron transfer and energy transfer is proposed based on the results of ESI-MS analysis, cyclic voltammetry, Stern–Volmer quenching experiments and control experiments. The photoluminescence properties (emission spectra and quantum yields) of the obtained indeno-coumarins were investigated.


 Please wait while we load your content...
Please wait while we load your content...