A chiral auxiliary-based synthesis of the C5–C17 trans-decalin framework of anthracimycin†
Abstract
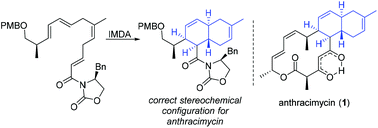
Synthetic studies towards the potent antimicrobial natural product anthracimycin, including the successful synthesis of the trans-decalin fragment through an endo-selective intramolecular Diels–Alder reaction are reported. The synthesis utilised a highly E-selective Julia–Kocienski olefination to access a stable skipped triene intermediate which provided access to four cycloaddition precursors for comprehensive Diels–Alder investigation. Incorporation of a chiral Evans’ oxazolidinone auxiliary overrode the inherent facial selectivity of the Diels–Alder reaction. These synthetic studies provide a platform for further investigation of this natural product as a novel chemical scaffold for antimicrobial drug discovery.


 Please wait while we load your content...
Please wait while we load your content...