Rigid hindered N-heterocyclic carbene palladium precatalysts: synthesis, characterization and catalytic amination†
Abstract
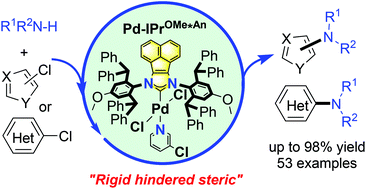
To explore the high efficiency of the Buchwald–Hartwig amination with a wide substrate scope, easily prepared and air stable palladium precatalysts bearing rigid hindered N-heterocyclic carbenes (NHCs) were synthesized and characterized. A simple and efficient protocol for the amination of (hetero)aryl chlorides with amines is described, which revealed that sterically encumbered NHC ligands are crucial to promote the transformation. It is highlighted that the most challenging reactions could be performed between less reactive five-membered heteroaryl chlorides and heteroanilines. This methodology provides a rapid and straightforward access to a wide range of arylated amines with excellent functional group tolerance. Remarkably, the powerful synthetic utility of palladium precatalysts was further extended to the synthesis of pharmaceuticals.


 Please wait while we load your content...
Please wait while we load your content...