Regular tuning of the ESIPT reaction of 3-hydroxychromone-based derivatives by substitution of functional groups†
Abstract
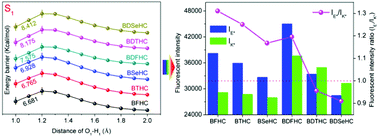
The excited state intramolecular proton transfer (ESIPT) reaction and photophysical properties of a series of 3-hydroxychromone (HC)-based derivatives (BFHC, BTHC, BSeHC, BDFHC, BDTHC and BDSeHC) were explored systematically using density functional theory (DFT) and time-dependent DFT (TD-DFT) methods. The enhanced excited-state intramolecular hydrogen bonds of the studied molecules contributed to the ESIPT reaction according to hydrogen-bond parameters and infrared-vibration analyses. The various substituent groups induced regular multi-channel signal changes (wavelength and intensity) of the fluorescence spectra of molecules in enol and keto forms. The potential energy curves showed the energy barriers in the S1-state of the molecules followed the order BFHC < BTHC < BSeHC and BDFHC < BDTHC < BDSeHC. Combination of topology analyses of the bond critical point present in the molecules revealed that BFHC and BTHC would show an ultrafast ESIPT reaction, whereas the other molecules would have a slower ESIPT reaction. The weakened electron-withdrawing ability of an atom and longer substituent group would frustrate the ESIPT reaction of the studied molecules, thereby affecting their photophysical properties. These novel discoveries can extend the application of HC derivatives in ESIPT-based optical devices.


 Please wait while we load your content...
Please wait while we load your content...