1,3-Dipolar cycloaddition of nitrones to oxa(aza)bicyclic alkenes†
Abstract
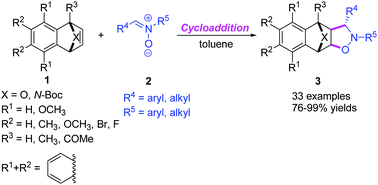
A new 1,3-dipolar cycloaddition of oxa(aza)bicyclic alkenes with nitrones has been developed. The operationally simple cycloaddition allows efficient synthesis of fused bicyclic tetrahydroisoxazoles bearing exo- and anti-five member heterocyclic rings (76–99% yields) without any catalyst and additive under mild conditions. The proposed concerted mechanism and the observed selectivity are investigated by DFT calculations of the reaction pathways. This mild and practical [3 + 2] cycloaddition provides an efficient route to access highly functionalized compounds.


 Please wait while we load your content...
Please wait while we load your content...