Phosphine-catalyzed fixation of CO2 with γ-hydroxyl alkynone under ambient temperature and pressure: kinetic resolution and further conversion†
Abstract
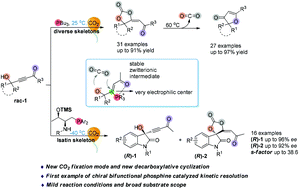
Multiple skeleton derived γ-hydroxyl alkynones could be activated by phosphine, which then underwent cycloaddition with CO2 to afford functionalized carbonate products under ambient temperature and pressure. These functionalized carbonate products could easily release CO2 under heating conditions, giving a diversity of furanones in excellent yields. The optically active functionalized cyclic carbonates could be afforded through the kinetic resolution of propargyl alcohols via carbon dioxide fixation catalyzed by a new series of sterically hindered and highly nucleophilic bifunctional amino acid-derived phosphine catalysts with moderate to excellent selectivities. Plausible mechanisms were proposed and supported by isotope-labeling experiments and DFT calculations.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...