A theoretical study of the ESIPT mechanism of 3-hydroxyflavone derivatives: solvation effect and the importance of TICT for its dual fluorescence properties
Abstract
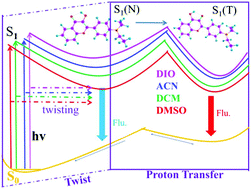
In this paper, the excited-state intramolecular proton transfer (ESIPT) process and the solvatochromic effect of 2-(4-(diethylamino)phenyl)-3-hydroxy-4H-chromen-4-one (DHC) molecules were reported by the density functional theory and time-dependent density functional theory method. We optimized the structures in four kinds of solvents, and the recorded absorption and fluorescence spectra were in agreement with the experiments. By establishing the potential energy curve, we proved that this fluorescent probe was designed based on the ESIPT process of the parent molecule DHC. A deeper study of the ESIPT process revealed that the energy consumed by the molecules along the C5–C18 bond twist was an important part of the dual fluorescence characteristics of the DHC, and the geometric structure tended to be planar with the decrease of the dielectric constant of the solvent. The effect of solvent media on electronic transition energies and absorption as well as florescence spectra were presented. As we know, a driving force of the proton transfer reaction was provided by hydrogen bonding. By comparing the infrared vibration spectrum, bond length and bond angle, we found that the hydrogen bond was enhanced after photoexcitation and was gradually increased as the solvent dielectric constant decreased (dimethylsulfoxide (DMSO) < acetonitrile (ACN) < dichloromethane (DCM) < 1,4-dioxane (DIO)), which was also evidenced by the reduced density gradient function (RDG) isosurface and scatter plots. Therefore, the process of ESIPT reaction was controlled by the solvent. Furthermore, the effect of the solvent on ESIPT was explained by comparing the reaction energy barrier in four kinds of solvents. The results showed that ESIPT was more likely to occur as the solvent dielectric constant decreased.


 Please wait while we load your content...
Please wait while we load your content...