Iron-mediated site-selective oxidative C–H/C–H cross-coupling of aryl radicals with quinones: synthesis of β-secretase-1 inhibitor B and related arylated quinones†
Abstract
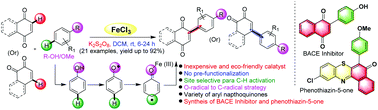
Phenoxy radicals generated from substituted arenes were converted into para site selective C-aryl radicals and coupled with quinones, using an inexpensive FeCl3–K2S2O8 system, to obtain several arylated quinones, in good to moderate yields, under operationally simple and mild conditions. This method is useful for one pot synthesis of β-secretase-1 (BACE) inhibitor B. The arylated quinones were used as intermediates in the synthesis of phenothiazin-5-ones. Theoretical studies on the pharmacokinetic properties of phenothiazin-5-ones showed high lipophilicity (log p = 3.69), poor water solubility (log s = −6.42), and high gastrointestinal absorption (GI).


 Please wait while we load your content...
Please wait while we load your content...