Utilization of nitriles as the nitrogen source: practical and economical construction of 4-aminopyrimidine and β-enaminonitrile skeletons†
Abstract
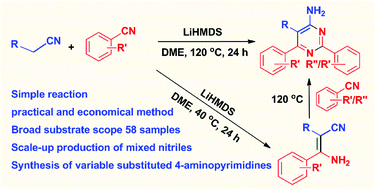
A highly practical and economical method for the synthesis of 4-aminopyrimidines and β-enaminonitriles by condensation of mixed organonitriles is reported. Both reactions are promoted by lithium hexamethyldisilazide (LiHMDS). The metal-free transformation process is suitable for scale-up production. The influence of bases, solvents, and substituents on the aromatic rings is studied. The scope of the method has been successfully demonstrated with 58 examples. Mechanistic studies revealed the existence of β-enaminonitriles as intermediates to make 4-aminopyrimidines. A mechanism of the cycloaddition process is proposed including the production of β-enaminonitriles and 4-aminopyrimidines, respectively, by means of controlling reaction temperature.


 Please wait while we load your content...
Please wait while we load your content...