Catalyst- and additive-free annulation/aromatization leading to benzothiazoles and naphthothiazoles†
Abstract
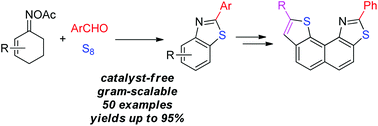
A catalyst- and additive-free three-component assembly of cyclohexenone oximes, aldehydes, and elemental sulfur has been developed. This protocol provides a highly efficient entry to naphtho[1,2-d]thiazoles and benzo[d]thiazoles with a broad range of functionalities tolerated.


 Please wait while we load your content...
Please wait while we load your content...