Regioselective C–H alkylation and alkenylation at the C5 position of 2-amino-1,4-naphthoquinones with maleimides under Rh(iii) catalysis†
Abstract
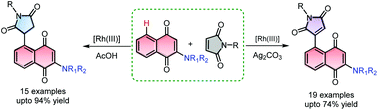
Rhodium(III) catalyzed regioselective C–H alkylation and alkenylation at the C5 position of 1,4-naphthoquinone with maleimides under acidic and basic conditions, respectively, is described. The alkylamino substituents at the C2 position enabled the reaction to proceed efficiently. The alkylation reaction proceeds via a redox neutral Rh(III) pathway, whereas the alkenylation reaction takes place through the typical redox Rh(III) pathway to yield Rh(I) species.


 Please wait while we load your content...
Please wait while we load your content...