Asymmetric synthesis of polycyclic 3-fluoroalkylproline derivatives by intramolecular azomethine ylide cycloaddition†
Abstract
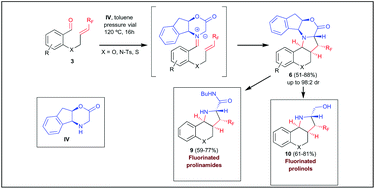
The asymmetric synthesis of polycyclic fluorinated proline derivatives has been accomplished by means of an intramolecular azomethine ylide cycloaddition with fluorinated dipolarophiles. Thus, the reaction of benzaldehydes bearing a trifluoromethyl alkene in ortho position with an azomethine ylide precursor derived from chiral 2-amino indanol takes place with excellent levels of diastereoselectivity, with simultaneous generation of four stereocenters. In order to determine the influence of the fluorine moiety in the process, starting material bearing non-fluorinated alkenes were synthesised. In this case, a dramatic drop of selectivity occurred, which indicates that the role of the fluorine substituent is crucial to achieve the observed selectivities. Theoretical calculations performed in model substrates agreed completely with experimental results. Finally, cycloaddition products were derivatised to the corresponding proline derivatives in a very simple manner.


 Please wait while we load your content...
Please wait while we load your content...