Organic photoredox catalytic decarboxylative cross-coupling of gem-difluoroalkenes with unactivated carboxylic acids†
Abstract
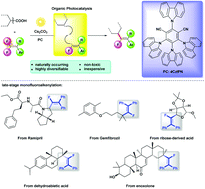
The monofluoroalkene substructure plays a crucial role in various fields of organic synthesis, pharmacology and materials science. However, a redox-neutral direct monofluoroalkenylation of prevalent alkyl sources still remains very challenging. Herein, the first monofluoroalkenylation of naturally abundant unactivated carboxylic acids via organic photoredox catalytic decarboxylation has been developed. This redox-neutral approach avoids the utilization of presynthesized redox-active esters and high energy irradiation, providing a mild and efficient strategy for direct decarboxylative cross-coupling reactions of unactivated carboxylic acids. Furthermore, it allows the remarkable and challenging late-stage monofluoroalkenylation of complex molecular architectures such as bioactive ramipril, gemfibrozil, dehydroabietic acid, enoxolone and saccharide-derived cyclic α-oxy acid.


 Please wait while we load your content...
Please wait while we load your content...