Copper-promoted dehydrogenative cross-coupling reaction of dialkyl phosphites with sulfoximines†
Abstract
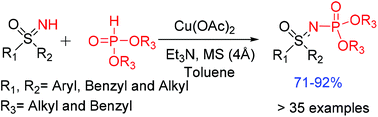
An efficient method for the dehydrogenative cross-coupling of dialkyl phosphites with sulfoximines is demonstrated in the presence of copper(II) acetate and triethylamine. A library of sulfoximines including aryl, heteroaryl and alkyl sulfoximines underwent coupling reaction smoothly and gave the desired sulfoximine derived phosphoramidates in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...