Synthesis and antiproliferative activities of OSW-1 analogues bearing 2-acylamino-xylose residues†
Abstract
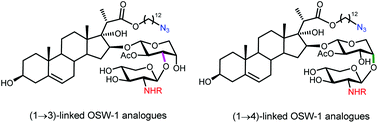
OSW-1 and SBF-1 are well studied saponins with exceptionally potent antitumor activities. Herein, we report the syntheses of a library of 38 C22-ester analogues bearing 2-acylamino xylose residues. SAR studies show that the introduction of the 2-acylamino xylose residues could further increase the antitumor activities as many as 40 folds than those of SBF-1 and the (1→3)-disaccharide linkage is crucial to the activities. A highly potent probe (3) bearing photoactivatable and clickable residues has been identified.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...