Orthogonally arranged tripyrrin–BODIPY conjugates with an “edge to plane” mode†
Abstract
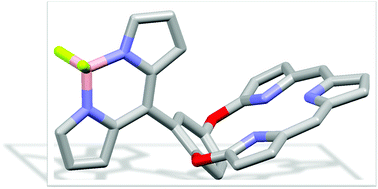
We designed new molecular conjugates, consisting of two strong fluorophores, tripyrrin and BODIPY moieties. Crystal structures demonstrated that the BODIPY is nearly perpendicular to the tripyrrin plane, directly linked in an orthogonal geometry, in a new “edge to plane” mode with covalently bonded chromophore peripheries. Furthermore, substitutions on BODIPY can modulate the photophysical properties, which can be explained by energy transfer via a FRET mechanism. This not only enriches the repertoire of multichromophoric system but also expands the scope of tripyrrin for constructing molecular functional materials.


 Please wait while we load your content...
Please wait while we load your content...