Iridium-catalyzed asymmetric hydrogenation of quinazolinones†
Abstract
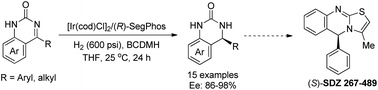
Enantioselective hydrogenation of quinazolinones has been successfully realized by employing a chiral iridium/diphosphine complex as catalyst, furnishing the chiral dihydroquinazolinones with excellent yield and up to 98% enantioselectivity. Asymmetric hydrogenation at the gram scale was also conducted smoothly without loss of reactivity and enantioselectivity. Using the above methodology as the key step, the enantiopure bioactive Eg5 inhibitor and (−)-SDZ 267-489 could also be conveniently synthesized.


 Please wait while we load your content...
Please wait while we load your content...