An unexpected reaction of aryldiazonium tetrafluoroborates, sodium metabisulfite, and thiourea under photoinduced conditions†
Abstract
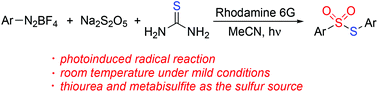
A photoinduced synthesis of S-aryl thiosulfonates through a three-component reaction of aryldiazonium tetrafluoroborates, sodium metabisulfite, and thiourea is achieved. The reaction scope generality with a range of aryldiazonium tetrafluoroborates is demonstrated. In this transformation, a radical coupling pathway is proposed with the insertion of sulfur dioxide in the presence of a photocatalyst under visible light irradiation. The organic sulfur motifs in S-aryl thiosulfonates originate from the convenient, cheap, and easily available thiourea and sodium metabisulfite.


 Please wait while we load your content...
Please wait while we load your content...