Asymmetric total synthesis of (+)-astellatol and (−)-astellatene†
Abstract
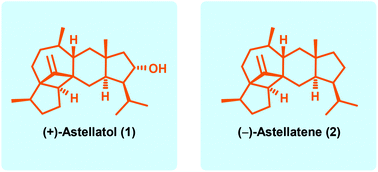
The chemical structure of isopropyl trans-hydrindane sesterterpenoids features a highly substituted trans-hydrindane moiety that holds an isopropyl or isopropenyl group at the segment to the right and a congested polycyclic ring system at the segment to the left, therefore representing formidable challenges for their synthesis. Here, we wish to describe the full account of our synthesis of (+)-astellatol, as well as the first total synthesis of (−)-astellatene. Our work highlights a mercury(II)-catalyzed Grignard reaction, an intramolecular Pauson–Khand reaction, a samarium(II)-mediated reductive radical 1,6-addition and a novel strategy for the synthesis of highly substituted trans-hydrindane natural products.


 Please wait while we load your content...
Please wait while we load your content...