Copper-catalyzed highly diastereoselective cross-dehydrogenative coupling between 8-hydroxyisochromanes and 1,3-dicarbonyl compounds†
Abstract
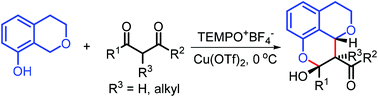
Herein, a novel copper-catalyzed highly diastereoselective cross-dehydrogenative coupling reaction is reported. A broad range of 1,3-dicarbonyl compounds and 8-hydroxyisochromanes are applicable to this methodology, affording tricyclic chromane products in moderate to high yields with continuous stereocenters identical to natural penicitrinols.


 Please wait while we load your content...
Please wait while we load your content...