Bimolecular oxidative C–H alkynylation of α-substituted isochromans†
Abstract
A bimolecular oxidative C–H functionalization of secondary benzylic ethers for new C–C bond formation has been established. A wide range of α-substituted isochromans underwent oxidative C–H alkynylation with diverse potassium trifluoroborates, affording respective oxygen heterocycles bearing α-tetrasubstituted stereocenters in high efficiency.
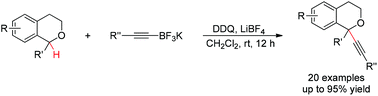


 Please wait while we load your content...
Please wait while we load your content...