A AgOAc/quinine-derived aminophosphine complex as an efficient catalyst for diastereo- and enantioselective 1,3-dipolar cycloaddition of α,β-unsaturated 7-azaindoline amides and azomethine ylides†
Abstract
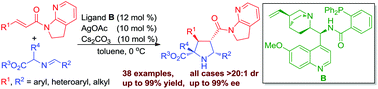
A quinine-derived aminophosphine/Ag(I) complex was firstly demonstrated to be an efficient catalyst for the highly stereoselective 1,3-dipolar cycloaddition of azomethine ylides with challenging α,β-unsaturated amide substrates. A wide range of densely functionalized pyrrolidine derivatives containing four contiguous stereogenic centers could be smoothly obtained with excellent diastereo- and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...