Carboxylate phosphabetaine as a bifunctional organocatalyst for the intramolecular ring opening of oxetane†
Abstract
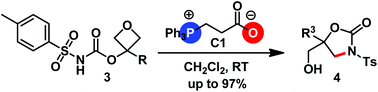
Herein, we disclose that carboxylate phosphabetaine can act as a competent organocatalyst for promoting the intramolecular ring opening of oxetanes, delivering oxazolidin-2-ones in good to excellent yields. 1H NMR studies and DFT calculations revealed that the carboxylate moiety of the phosphabetaine not only acts as a proton shuttle, but also provides crucial hydrogen bonding for the activation of the oxetane ring.


 Please wait while we load your content...
Please wait while we load your content...