A bifunctional pyrazolone–chromone synthon directed organocatalytic double Michael cascade reaction: forging five stereocenters in structurally diverse hexahydroxanthones†
Abstract
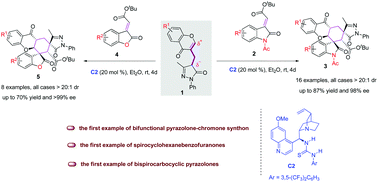
The merging of two or more known natural product-based scaffolds is a powerful and routine strategy to develop bioactive small molecules. Here, we wish to report the first example of the bifunctional pyrazolone–chromone synthon 1 directed organocatalytic double Michael tandem reaction, which serves as a fruitful strategy for the facile access to structurally diverse hexahydroxanthone derivatives 3 and 5 bearing five continuous stereocenters, including two all-carbon quaternary spiro-stereocenters. These products were smoothly afforded in up to 87% yield, >20 : 1 dr and >99% ee under mild conditions. This is also the first example of catalytic enantioselective access to spirocyclohexanebenzofuranones and bispirocarbocyclic pyrazolones, potentially useful in medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...