A biomimetic strategy for the selective recognition of organophosphates in 100% water: synergies of electrostatic interactions, cavity embedment and metal coordination†
Abstract
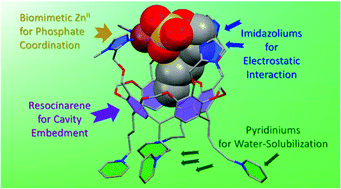
Phosphate recognition in water is a hot topic due to their importance in biology and environment. The design and conception of water-soluble receptors that are efficient and selective is a difficult task because of the water medium, which provides highly competitive electrostatic interactions. A possible strategy relies on multipoint recognition with a polycationic site for electrostatic interaction with the phosphate moiety and a hydrophobic cavity for the organic one. Following this strategy, a detailed study of the hosting properties of a water-soluble resorcinarene-based metallo-cavitand, WRim4Zn, with various organophosphates is presented. The ZnII receptor showed good affinity and selectivity for n-alkyl phosphates (CnP) having from 2 to 6 carbons, in 100% water at physiological pH. The strongest association was obtained with C6P [K′pD7.4 = 3.6 × 104 M−1] whereas C7P binds 100 times less strongly. This very sharp selectivity pattern stands in strong contrast with carboxylate guests for which the optimum size corresponds to acetate. The different selectivity pattern stems from a switch in the coordination mode of ZnII upon phosphate binding, inducing the decoordination of two out of the four imidazole arms grafted on the resorcinarene macrocycle. Hence, the efficiency of WRim4Zn for alkyl phosphate recognition and unusual selectivity relative to the organic moiety of the phosphate analytes is ascribed to the synergistic action of a metal binding site for the phosphate head (the ZnII receptor is 100 times more efficient than the cavity ligand WRim4 itself), a hydrophobic cavity for alkyl embedment, protonated arms leading to further electrostatic stabilization, spacers between the recognition points (ZnII, imidazoliums, resorcinarene bowl cavity) allowing directional and spatial control of guest binding. Another key point is the water-solubility of the hydrophobic core insured by the pyridinium feet positioned away from the receptacle, which avoids the destructuring of the receptor metallo-site. Phosphodiesters, which are singly charged and congested, are not recognized. Hence, the good affinity displayed for C6P makes WRim4Zn one of the best cavity-based receptor for organophosphates described to date. These results open new routes for sensing biologically and environmentally important organic anions, considering the tunability of the functionalization pattern of the resorcinarene core.
- This article is part of the themed collection: In celebration of Julius Rebek’s 75th Birthday


 Please wait while we load your content...
Please wait while we load your content...