The absolute configurations of hyperilongenols A–C: rare 12,13-seco-spirocyclic polycyclic polyprenylated acylphloroglucinols with enolizable β,β′-tricarbonyl systems from Hypericum longistylum Oliv.†
Abstract
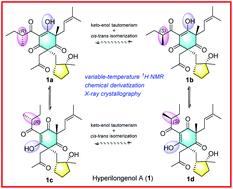
Three novel 12,13-seco-spirocyclic polycyclic polyprenylated acylphloroglucinol (PPAP) derivatives, named hyperilongenols A–C (1–3), were isolated from the stems and leaves of Hypericum longistylum Oliv. Their planar structures were established by a combination of 1D and 2D NMR, HRESIMS data, and chemical derivatization. Their absolute configurations were defined using electronic circular dichroism (ECD) spectroscopy and X-ray crystallography. Compounds 1–3 represent the first examples of naturally occurring 12,13-seco-spirocyclic PPAPs with an enolizable β,β′-tricarbonyl system. The O-methylation of compounds 1–3, coupled with comprehensive analyses of their X-ray diffraction data and variable-temperature 1H NMR spectra collected in different solvents, allowed us to propose a general mechanism underlying the formation of inseparable C-24 epimers of hyperilongenol A (1), decipher the solvent effects on the enolizable β,β′-tricarbonyl enol protons, and explore the possibilities of keto–enol interconversions of compounds 1–3. Moreover, a reasonable proposal for the structural determination of PPAPs with enolizable β-dicarbonyl and β,β′-tricarbonyl systems was developed. In addition, an in vitro bioassay showed that 2 exhibited potent antibacterial activity against Staphylococcus aureus.


 Please wait while we load your content...
Please wait while we load your content...