A hexameric resorcinarene capsule as a hydrogen bonding catalyst in the conjugate addition of pyrroles and indoles to nitroalkenes†
Abstract
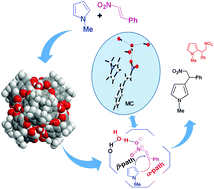
A hexameric resorcinarene capsule acts as a hydrogen bonding catalyst for the activation of encapsulated nitroalkenes toward the addition of pyrroles and indoles. Once inside the capsule (C), β-nitrostyrene 3a establishes H-bonding interactions with the bridging water molecules having free H-bond-donating valences. Thus, the activation of nitroalkenes favors the conjugate addition of pyrroles and indoles. It is demonstrated that the inherent acidity of capsule C is sufficient to promote the Michael-type Friedel–Crafts (MTFC) reaction under the mild reaction conditions described herein. In silico calculations point out the catalytically relevant role of the bridging water molecules of C and confirm the supramolecular control exerted by the capsule, which protects the nitronic acid intermediate with respect to the degradation pathway that commonly occurs in the bulk medium.


 Please wait while we load your content...
Please wait while we load your content...