Abstract
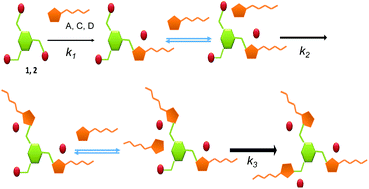
A strong preference for the formation of tripodal systems over the related monotopic and ditopic compounds is observed for the reaction between tris(halomethyl)benzenes and imidazoles derived from amino acids and containing an amide fragment. This preference allows the formation of the tripodal derivative as the major product even when an equimolar mixture of the tris(halomethyl)benzene and the imidazole is reacted (1 : 1 ratio instead of the stoichiometric 1 : 3 ratio). The reactions were monitored using 1H NMR spectroscopy and ESI mass spectrometry and kinetically characterized. Computational studies were also performed in order to rationalize the observed preference of the tri-substituted product. The results reveal the existence of well-defined supramolecular interactions between the imidazolium groups and the reacting imidazoles that facilitate the formation of the multitopic systems once the first imidazolium group is formed. Analysis of the different structural components shows that the presence of the amide group from the amino acid moiety is the key structural requirement for such supramolecular assistance to take place. The preorganization of the supramolecular intermediates formed through hydrogen bonding interactions involving amide-NH fragments in imidazoles and bromide anions in imidazolium groups seems to be also present at the corresponding TSs, decreasing the associated energy barriers.
- This article is part of the themed collection: In celebration of Julius Rebek’s 75th Birthday


 Please wait while we load your content...
Please wait while we load your content...