Abstract
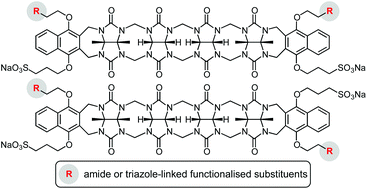
Synthetic strategies are described to prepare acyclic cucurbituril derivatives with two different types of appended functional groups, one that mediates water solubility and another one that allows further functionalisation. The syntheses started with the coupling of a 1,4-disubstituted naphthalene derivative containing one 2-chloroethoxy and one 3-sulfonatopropoxy group to a suitable tetrameric glycoluril-derived precursor. This reaction afforded two regioisomers, differing in the relative orientation of the peripheral substituents, which could be separated in a straightforward fashion and structurally characterised. Both isomers were then converted into the corresponding diazides and diamines that served to append further residues by, respectively, copper(I)-catalysed azide–alkyne cycloaddition or amide formation. Binding studies showed that the functionalised dianionic acyclic cucurbiturils thus obtained possess a notable cation affinity in water, albeit a lower one than an analogue with four peripheral negatively charged substituents. This work constitutes the basis for the development of water-soluble acyclic cucurbiturils whose applications could potentially go beyond the use as receptors.
- This article is part of the themed collection: In celebration of Julius Rebek’s 75th Birthday


 Please wait while we load your content...
Please wait while we load your content...