Synthesis, characterization and crystal structures of novel fluorinated di(thiazolyl)benzene derivatives†
Abstract
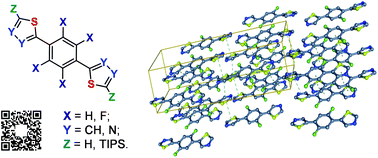
A series of 11 novel fluorinated and non-fluorinated di(thiazolyl)benzenes have been synthesized via microwave assisted Stille coupling and characterized using X-ray crystallography. A comparative analysis of nonbonding interactions in bis-heteroaryl-phenylene derivatives depending on the heterocycle structure and degree of fluorination of the central aromatic unit has been reported. We have systematically studied the effect of fluorination on the intramolecular interactions, π–π stacking, CH⋯F and S⋯F non-covalent interactions. Axial conformations of the biaryl system A is regulated by thermodynamic fluctuations and steric repulsions. On the other hand 2-phenyl thiophene B consists of only one H,H-repulsion interaction with a possibility of having a weak noncovalent CH⋯S interaction, hence, the combination of these factors results in the decrease in the energetics of the eclipsed conformation. When replacing the thiophene moiety with the thiazol-2-yl substituent C this allow us to eliminate the H,H-repulsions and results in CH⋯N hydrogen bonding and CH⋯S interactions. S⋯F non-covalent interactions can be used for pre-orientation of organic conjugated materials in a quasi-planar conformation.


 Please wait while we load your content...
Please wait while we load your content...