Reactions of 2-aza-21-carbaporphyrin with aniline derivatives†
Abstract
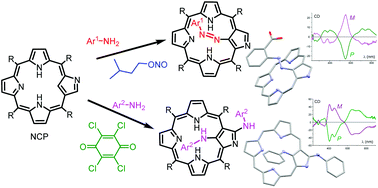
Regioselective azotization or bis(amination) products of the title porphyrinoid were achieved in one-pot one-step syntheses under mild conditions using a selection of aromatic amines and isoamyl nitrite or p-chloranil, respectively. Structures, tautomery, chirality as well as spectroscopic and redox properties of the new systems were discussed.


 Please wait while we load your content...
Please wait while we load your content...