A copper-catalyzed radical coupling/fragmentation reaction: efficient access to β-oxophosphine oxides†
Abstract
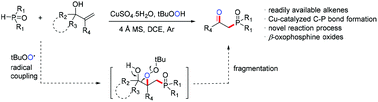
Described herein is a novel and efficient copper-catalyzed oxidative phosphonation of α-tert-hydroxylalkenes. This protocol displays a broad scope with good functional group compatibility and gives efficient access to diverse β-oxophosphine oxides from simple allylic alcohols and H-phosphine oxides. The mechanism is proposed to be an unprecedented three-component radical coupling/fragmentation cascade reaction of α-tert-hydroxylalkenes, HP(O)R1R2 and tert-butyl hydroperoxide (TBHP), in which the excess TBHP acts as not only a radical initiator but also a coupling partner.


 Please wait while we load your content...
Please wait while we load your content...