Carbene-catalyzed oxidative acylation promoted by an unprecedented oxidant CCl3CN†
Abstract
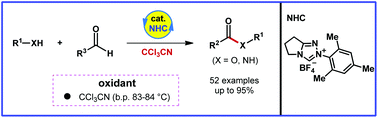
An unprecedented example of a NHC-catalyzed acylation reaction promoted by an oxidant CCl3CN is described. This protocol features several advantages, including mild reaction conditions, broad substrate scope, and easy operation. In addition, low cost, low boiling point, and small molecular weight allow CCl3CN to be an attractive and amenable oxidant. Meanwhile, this formal dehydrogenative coupling reaction of aldehydes and alcohols involves a hydride transfer process.


 Please wait while we load your content...
Please wait while we load your content...