Manganese-mediated reductive amidation of esters with nitroarenes†
Abstract
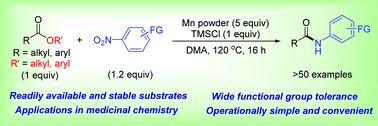
Amides are ubiquitous molecules in nature and in synthetic chemistry. Here we report a convenient and efficient method to synthesize N-aryl amides via amidation of esters with nitroarenes. In the presence of manganese metal, this amidation proceeded smoothly without the need for additional catalysts or ligands. Various esters and nitroarenes are suitable substrates to afford a wide range of N-aryl amides, including bio-active molecules and intermediates to drug molecules.


 Please wait while we load your content...
Please wait while we load your content...