Fischernolides A–D, four novel diterpene-based meroterpenoid scaffolds with antitumor activities from Euphorbia fischeriana†
Abstract
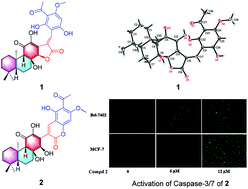
Fischernolides A–D (1–4), four meroterpenoids based on diterpene and acylphloroglucinol, possessing an unprecedented 28-carbon skeleton with a novel scaffold, were isolated from the roots of Euphorbia fischeriana. Their structures and absolute configurations were determined by comprehensive chemical analyses including spectroscopic techniques, electronic circular dichroism (ECD) spectroscopy, and single crystal X-ray diffraction experiments. The possible biogenetic pathway to generate 1–4 through aldol condensation was also proposed. Compound 2 showed significant cytotoxicity and can induce the apoptosis of MCF-7 and Bel-7402 cell lines by caspase activation.


 Please wait while we load your content...
Please wait while we load your content...