Enantioselective synthesis of 3-amino-hydrobenzofuran-2,5-diones via Cu(i)-catalyzed intramolecular conjugate addition of imino esters†
Abstract
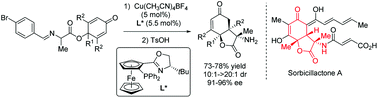
A highly enantioselective intramolecular conjugate addition of imino esters was presented. By employing cyclohexadienone-tethered imino esters, enantioenriched 3-amino-hydrobenzofuran-2,5-dione skeletons bearing three contiguous stereocenters were synthesized via the desymmetrization of prochiral cyclohexadienones. Notably, bicyclic cyclohexenones bearing up to three vicinal quaternary stereocenters could be constructed in a single step by this strategy.
- This article is part of the themed collection: 2019 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...