Total synthesis of giffonin H by fluoride-catalyzed macrocyclization†
Abstract
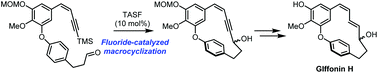
The first total synthesis of giffonin H, a highly strained 15-membered macrocyclic diaryl ether, has been achieved in eight linear steps with 10% overall yield. Key steps include Ullmann cross coupling, (Z)-selective Julia–Kocienski olefination, and fluoride-mediated macrocyclization of TMS-alkyne and aldehyde. In particular, the strategy used for macrocyclization is an unprecedented and unique synthetic approach for cyclic diarylheptanoids. The fluoride-mediated macrocyclization strategy should be applicable to the synthesis of giffonin congeners under mild reaction conditions without affecting the integrity of subtle diene moieties.


 Please wait while we load your content...
Please wait while we load your content...