Nickel-promoted C(2)–H amidation of quinoline N-oxides with N-fluorobenzenesulfonimide†
Abstract
Reported herein is an unprecedented nickel-promoted C–H amidation reaction of quinoline N-oxides using N-fluorobenzenesulfonimide (NFSI) as the amination reagent. This strategy provides a convenient access to a variety of useful 1,2-dihydro-2-iminoquinolines in moderate to good yields. Notable advantages of this protocol include easy operation and mild reaction conditions.
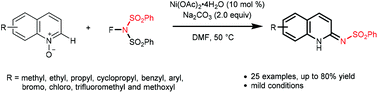


 Please wait while we load your content...
Please wait while we load your content...