Trisannulation of benzamides and cyclohexadienone-tethered 1,1-disubstituted allenes initiated by Cp*Rh(iii)-catalyzed C–H activation†
Abstract
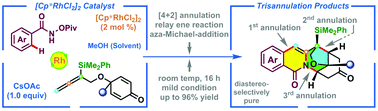
A diastereoselectively pure trisannulation of N-(pivaloyloxy)benzamides and newly synthesized cyclohexadienone-tethered 1,1-disubstituted allenes has been successfully established via the initiation of Cp*Rh(III)-catalyzed C–H bond activation. The tandem reaction, involving a formal Cp*Rh(III)-catalyzed [4 + 2]-annulation, a relay ene reaction, and an intramolecular aza-Michael-addition sequence, proceeds smoothly at room temperature to deliver the special trisannulation products with high to excellent yields (up to 96%). Furthermore, the gram-scale reaction and post-transformation are also presented.


 Please wait while we load your content...
Please wait while we load your content...